Lab: Stoichemetry Investigation- Testing the Stoichiometric Method
In this class we were presented with a problem: Does Stoichiometry accurately predict the mass of products produced in chemical reactions?
I was in a group of three along with Sherilyn and Christina.
Our prediction before we started or experiment was to first, find out the balanced chemical equation for this reaction: 2.00g of Strontium nitrate is dissolved in 50mL of water and then reacted with excess Copper (II) sulphate (3.00g). The product is a precipitate (Strontium sulphate and Copper (II) nitrate.
Our balanced equation was: Sr(NO
3)
2 + CuSO
4 ® SrSO
4 + CU(NO
3)
2
Secondly we had to find how much of Strontium sulphate would be produced from 2.00g of Strontium nitrate. Our answer was: 2.00g x
1 mol x
1 x
183.7g
211.6g 1 1 mol = 1.74g of SrSO
4
In the beginning of the experiment we had to carefully measure about 3.00g of Copper (II) sulphate and 2.00g of Strontium nitrate and dissolve them in separate beakers of 50mL of water. We slowly poured the two solutions together and mixed it. After pouring the solutions we had to measure a filter paper and filter the solution. Once finished we dried the filter paper and waited till dried.
Our results came out to be: a fail.
Our results will be completed soon...
~ Kelly
LESSON 23 Energy & Percent Yield
ENERGY
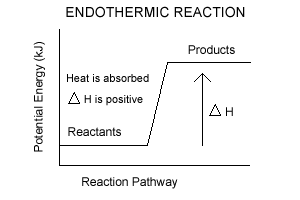
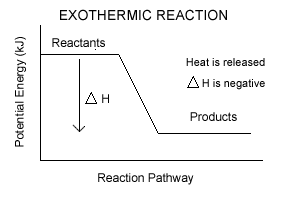
- Enthalpy is the energy stored in chemical bonds
- Symbol of Enthalpy is H
- units of joules (J)
- Change in Enthalpy is ΔH
- In exothermic rxns, enthalpy decreases
- In endothermic rxns, enthalypy increases
CALORIMETRY
- To experimentally determine the heat released, we need to know 3 things
- Temperature change (ΔT)
- Mass (m)
- Specific Heat Capaacity (CΝ = 4.19J / g °C)
These are related by the equation:
ΔH = mCΔT
Examples
Calculate the heat required to warm a cup of 500g of water (CΝ = 4.18J / g°C) from 20.0°C to 50.0 °C.
ΔH = mCΔT
= (500g)(4.18J / g°C) (50.0°C - 20.0°C)
= 62 700 J = 6.3 x 10^4 J = 62.7 kJ
PERCENT YIELD
- The theoretical yield of a reaction is the amount of products that SHOULD be formed.
- The actual amount depends on the experiment.
- The percent yield is like a measure of success
- How close is the actual amount to the predicted amount? % YIELD = Actual / Theoretical x 100
Examples
Production of Urea: 2NH3 + CO2 -> CO(NH2)2 + H2O
If 32.1g of Urea are produced, determine theoretical yield of CO2?
32.1g x [mol/60g] = 0.535mol x [1/1] x [44.0g/mol] = 23.5 g
What is the % yield of CO2 if 18.0g is produced?
[18.0g/23.0g] x 100 = 78%
LIMITING REACTIONS
Usually one chemical gets used up before the other
- The chemical used up first is called the limiting reactant
- Once it's used up the reaction stops
- L.R. determines the quantity of products formed
- To determine L.R. assume one reactant is used up. Determine how much of this reactant is required.
Find the L.R. when 2.8mol of H2 reacts with 1.25 mol of O2.
2H2 + O2 -> 2H2O
2.8mol x [1/2] = 1.4mol of O2 O2 is L.R!
1.24mol x [2/1] = 2.5mol of H2 O2 is L.R.!
★ kim