LESSON 23 Energy & Percent Yield
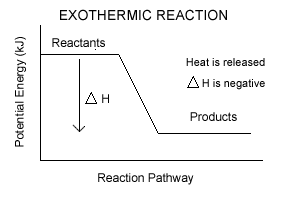
- Enthalpy is the energy stored in chemical bonds
- Symbol of Enthalpy is H
- Change in Enthalpy is ΔH
- In exothermic rxns, enthalpy decreases
- In endothermic rxns, enthalypy increases
CALORIMETRY
- To experimentally determine the heat released, we need to know 3 things
- Temperature change (ΔT)
- Mass (m)
- Specific Heat Capaacity (CΝ = 4.19J / g °C)
These are related by the equation:
ΔH = mCΔT
Examples
Calculate the heat required to warm a cup of 500g of water (CΝ = 4.18J / g°C) from 20.0°C to 50.0 °C.
ΔH = mCΔT
= (500g)(4.18J / g°C) (50.0°C - 20.0°C)
= 62 700 J = 6.3 x 10^4 J = 62.7 kJ
PERCENT YIELD
- The theoretical yield of a reaction is the amount of products that SHOULD be formed.
- The actual amount depends on the experiment.
- The percent yield is like a measure of success
% YIELD = Actual / Theoretical x 100
Examples
Production of Urea: 2NH3 + CO2 -> CO(NH2)2 + H2O
If 32.1g of Urea are produced, determine theoretical yield of CO2?
32.1g x [mol/60g] = 0.535mol x [1/1] x [44.0g/mol] = 23.5 g
What is the % yield of CO2 if 18.0g is produced?
[18.0g/23.0g] x 100 = 78%
LIMITING REACTIONS
Usually one chemical gets used up before the other
- The chemical used up first is called the limiting reactant
- Once it's used up the reaction stops
- L.R. determines the quantity of products formed
- To determine L.R. assume one reactant is used up. Determine how much of this reactant is required.
Find the L.R. when 2.8mol of H2 reacts with 1.25 mol of O2.
2H2 + O2 -> 2H2O
2.8mol x [1/2] = 1.4mol of O2 O2 is L.R!
1.24mol x [2/1] = 2.5mol of H2 O2 is L.R.!
★ kim
0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home